
The ionic character is due to shifting of the electron pair towards A or B in the molecule AB.
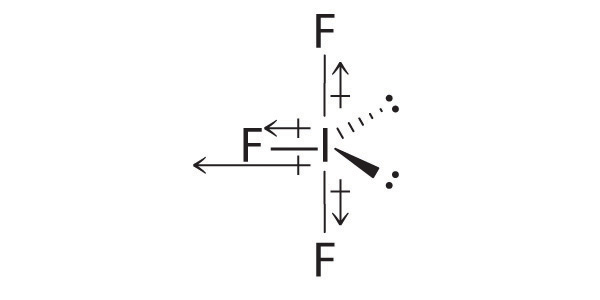
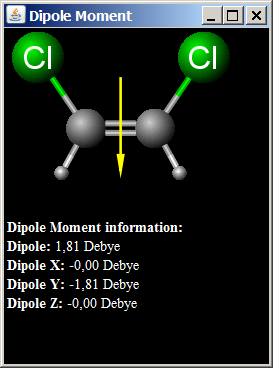
If the bond length is 1.0 xx 10^(-8) cm, what fraction of charge does exist on each atom?Ĭovalent molecules formed by heteroatoms bound to have some ionic character. The percentage of ionic character of a bond can be calculated by the application of the following formula : % " ionic character " = ("Experimental value of dipole moment ")/("Theoretical value of dipole moment ") xx 100 A diatomic molecule has a dipole moment of 1.2 D. Dipole moment values can be used to distinguish between cis-and traps-isomers, ortho-, meta-and para-forms of a substance, etc. Thus, dipole moments help to predict the geometry of the molecules. A symmetrical structure shows zero dipole moment. Hence, dipole moment of molecules depends upon the relative orientation of the bond dipoles, but not on the polarity of bonds alone. Dipole moment is equal to the product of charge separation, q and the bond length, d for the bond. Polar covalent molecules can exhibit dipole moment. Such a bond which has some ionic character is described as polar covalent bond. Hence, atoms acquire small and equal charge but opposite in sign. Covalent molecules formed by heteroatoms bound to have some ionic character.


 0 kommentar(er)
0 kommentar(er)
